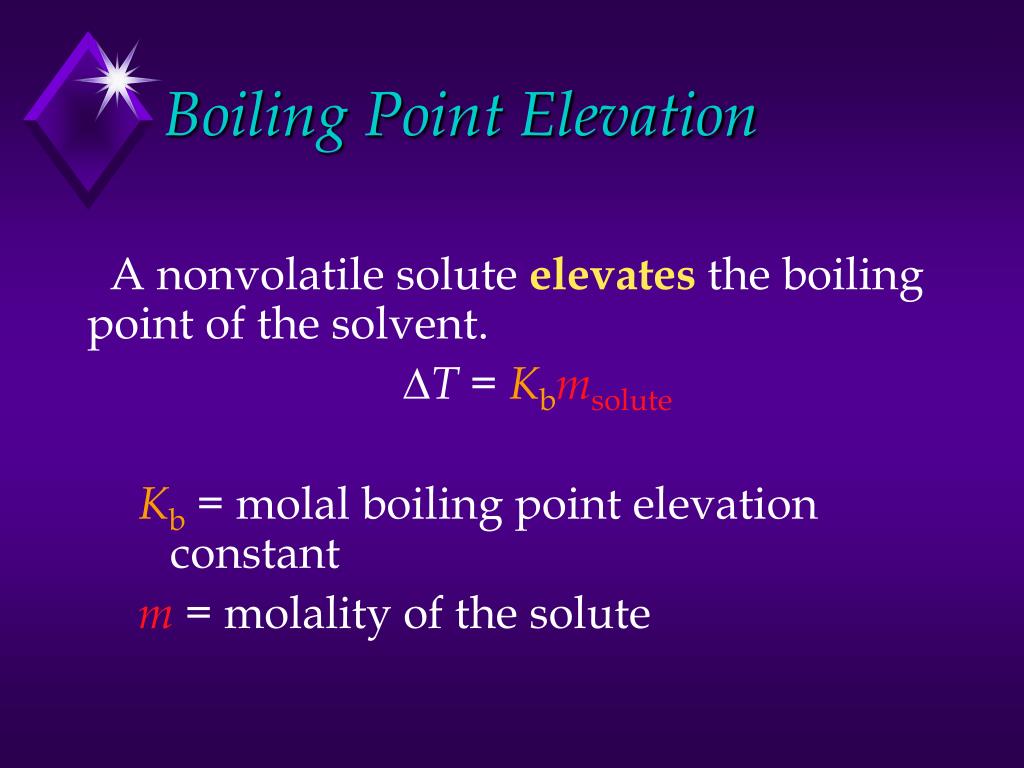
Boiling Point Elevation Syrup . Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. what is boiling point elevation? The objective of this experiment is to determine the freezing point depression. — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Solutions, boiling point elevation, colligative properties. thomas cahill and john wang. — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. For example, dissolving salt in water.
from www.slideserve.com
— the boiling point elevation is the amount that the boiling point temperature increases compared to the original. — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. The objective of this experiment is to determine the freezing point depression. — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. thomas cahill and john wang. — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. For example, dissolving salt in water. Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. Solutions, boiling point elevation, colligative properties. what is boiling point elevation?
PPT Overview of Ch 1113 PowerPoint Presentation, free download ID
Boiling Point Elevation Syrup what is boiling point elevation? For example, dissolving salt in water. what is boiling point elevation? Solutions, boiling point elevation, colligative properties. — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. thomas cahill and john wang. The objective of this experiment is to determine the freezing point depression. Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it.
From www.vrogue.co
Boiling Point Elevation Detailed Explanation With Exa vrogue.co Boiling Point Elevation Syrup Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. — the temperature at which the vapor pressure of a solution is 1 atm will be higher. Boiling Point Elevation Syrup.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Elevation Syrup what is boiling point elevation? — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. Solutions, boiling point elevation, colligative properties. For example, dissolving salt in water. Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. The objective of. Boiling Point Elevation Syrup.
From www.youtube.com
Boiling Point Elevation with Examples! YouTube Boiling Point Elevation Syrup The objective of this experiment is to determine the freezing point depression. — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Solutions, boiling point elevation, colligative properties. what is boiling point elevation? — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a. Boiling Point Elevation Syrup.
From www.youtube.com
12.02 Application of Boilingpoint Elevation YouTube Boiling Point Elevation Syrup — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Solutions, boiling point elevation, colligative properties. Boiling point elevation refers to the increase in the boiling. Boiling Point Elevation Syrup.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps Boiling Point Elevation Syrup Solutions, boiling point elevation, colligative properties. — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. what is boiling point elevation? thomas cahill and john wang. Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. — the. Boiling Point Elevation Syrup.
From www.youtube.com
Elevation In Boiling Point Class 12 // Solution / Part5 YouTube Boiling Point Elevation Syrup — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. Solutions, boiling point elevation, colligative properties.. Boiling Point Elevation Syrup.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and Boiling Point Elevation Syrup Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. Solutions, boiling point elevation, colligative properties. — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. The objective. Boiling Point Elevation Syrup.
From www.slideserve.com
PPT Overview of Ch 1113 PowerPoint Presentation, free download ID Boiling Point Elevation Syrup — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. Boiling point elevation refers to the. Boiling Point Elevation Syrup.
From slideplayer.com
How to Use This Presentation ppt download Boiling Point Elevation Syrup Solutions, boiling point elevation, colligative properties. — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as the boiling point elevation. Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. —. Boiling Point Elevation Syrup.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy Boiling Point Elevation Syrup For example, dissolving salt in water. — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. Solutions, boiling point elevation, colligative properties. — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. what is boiling point elevation? . Boiling Point Elevation Syrup.
From www.youtube.com
Elevation in Boiling Point Problems Solution and Colligative Boiling Point Elevation Syrup — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. For example, dissolving salt in water. what is boiling point elevation? The objective of this experiment is to determine the freezing point depression. thomas cahill and john wang. Solutions, boiling point elevation, colligative properties. — the temperature at. Boiling Point Elevation Syrup.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Boiling Point Elevation Syrup Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. Solutions, boiling point elevation, colligative properties. thomas cahill and john wang. — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. — the boiling point elevation (\(δt_b\)) and freezing. Boiling Point Elevation Syrup.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry Boiling Point Elevation Syrup Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. what is boiling point elevation? For example, dissolving salt in water. — the temperature at which the vapor pressure of a solution is 1 atm will be higher than the normal boiling point by an amount known as. Boiling Point Elevation Syrup.
From facts.net
20 Fascinating Facts About Boiling Point Elevation Boiling Point Elevation Syrup — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. what is boiling point elevation? thomas cahill and john wang. Solutions, boiling point elevation, colligative properties. Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute.. Boiling Point Elevation Syrup.
From www.youtube.com
Applications of Boiling Point Elevation and Freezing Point Depression Boiling Point Elevation Syrup Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. — the boiling point elevation is the amount that the boiling point temperature increases compared to the original. The objective of this experiment is to determine the freezing point depression. thomas cahill and john wang. — boiling. Boiling Point Elevation Syrup.
From www.chemicalslearning.com
Boiling Point ElevationDefinition, Formula with Examples Boiling Point Elevation Syrup — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. — boiling point elevation is the increase in the boiling point of a solvent by dissolving a nonvolatile solute into it. Solutions, boiling point elevation, colligative properties. thomas cahill and john wang. The objective of this. Boiling Point Elevation Syrup.
From www.studocu.com
Boiling Point Elevation Boiling Point Elevation What is the Boiling Point Elevation Syrup — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. Boiling point elevation refers to the increase in the boiling point of a solvent upon the addition of a solute. — the boiling point elevation is the amount that the boiling point temperature increases compared to the. Boiling Point Elevation Syrup.
From www.youtube.com
What is Boiling Point Elevation? YouTube Boiling Point Elevation Syrup For example, dissolving salt in water. thomas cahill and john wang. — the boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the. The objective of this experiment is to determine the freezing point depression. — the boiling point elevation is the amount that the boiling point temperature. Boiling Point Elevation Syrup.